Density of Aluminum – An Ultimate Guide
The density of aluminum is an essential fact that people should be aware of but might be unfamiliar with. A common issue faced by numerous industries is how to decide on which material to use because they fail to correctly interpret several parameters of aluminum, such as its density and its influence on the production. This can result in undesirable decisions concerning materials in the product design process which has an impact on the efficiency of the product as well as cost. Consider the irony of crafting a compact aircraft or vehicle and then realizing the material is not what you planned. To address these problems, here is the detailed look at the density of aluminum and its relevance given in an understandable manner.

Table of Contents
ToggleRevealing Insights On Density!
To get better understanding of the density of the aluminum, it is essential to uncover insights on the density. You can define it as the mass of substance in a givven amount of volume.
Units of Measurement for Density
| Sr. No. | Unit | Symbol | System |
| 1 | Kilogram per cubic meter | kg/m³ | SI |
| 2 | Gram per cubic centimeter | g/cm³ | CGS |
| 3 | Pound per cubic foot | lb/ft³ | Imperial |
| 4 | Pound per cubic inch | lb/in³ | Imperial |
| 5 | Ounce per cubic inch | oz/in³ | Imperial |
| 6 | Slug per cubic foot | slug/ft³ | Imperial |
| 7 | Ton per cubic yard | ton/yd³ | Imperial |
| 8 | Atomic units | a.u. | Atomic |
| 9 | Planck units | P | Planck |
| 10 | Imperial units | – | Imperial |
| 11 | Troy units | – | Troy |
| 12 | SI units | – | SI |
| 13 | CGS units | – | CGS |
Density of Aluminum!
Standard Density of Aluminum
Density shows how much material can be packed into a given space or volume. In the context of aluminum, density informs how compact it is, or how massive it is for a given volume. Aluminum density lies around 2.7 g/cm^3. In larger units or mass density of aluminum you can refer it as 27000 kg^m3. For instance of you take a cube of aluminum having one meter length on each side then it will be weight around 27000 kg. With this information you can have an idea on the desnity of aluminum considering the size.
| Sr. No. | Unit | Density of Aluminum |
| 1 | Kilogram per cubic meter | 2700 |
| 2 | Gram per cubic centimeter | 2.7 |
| 3 | Pound per cubic foot | 168.5 |
| 4 | Pound per cubic inch | 0.098 |
| 5 | Ounce per cubic inch | 1.57 |
| 6 | Slug per cubic foot | 0.097 |
| 7 | Ton per cubic yard | 0.87 |
| 8 | Atomic units | 1.05 × 10^24 |
| 9 | Planck units | 4.2 × 10^54 |
| 10 | Imperial units | 168.5 lb/ft³ |
| 11 | Troy units | 11.37 troy oz/cu in |
| 12 | SI units | 2700 kg/m³ |
| 13 | CGS units | 2.7 g/cm³ |
Factors Influencing the Density of Aluminum
Temperature
Density of aluminum changes due to the processes of thermal expansion as well as contraction due to temperature. Aluminum has the specific characteristic of becoming less dense as heat is applied, which is the outcome of expansion present in the material. On the other hand, cooling aluminum for example makes it shrink and at the same it becomes denser. This relationship is quite significant in applications where specific performance of the material is needed at different temperatures. Remember temperature when choosing an aluminum type for its application for high heat areas.
Alloy Composition
Some other ingredients found in the aluminum alloys are magnesium, silicon, or copper which contribute to differences in density. When a certain type of alloy is selected, the nature and concentration of these elements will affect the weight and performance of the whole material. Larger rounded elements in the mix add more weight or density and the smaller rounded elements lessen weight or density.
Impurities
The addition of other elements like iron or silicon makes aluminum slightly denser due to the extra mass they bring about in its purity.
The grade of aluminum depends with its purity as defined by the level of refining. What you will learn is that an increase in the purity grade means a lower density and a better performance in some of the most challenging applications that require lightweight.
Mechanical Processing
Such processes as rolling or forging, which may be used in the creation of aluminum products, can bring about changes in its weight density. In the case of aluminum, when force is applied in a particular direction, the material undergoes a change in the internal structure. This results in densification or sometimes voids depending on the type of method used. This in turn helps in the controlled processing of printing which helps in achieving uniform density and material strength.
Grain Size
Grain size in aluminum depends on the density and mechanical strength of the material’s properties. As the grain size becomes finer, the density increases and the durability is improved as well. This is specifically so when working on aluminum parts, which need strength; the smaller grain sizes help distribute loads and minimize chances of crack formation.
Phase Structure
Aluminum can be found in various phases depending on the heat and stress treatment of the material. Every phase has its own density. While using the aluminum material, you should consider how phase transformation like liquid to solid phase influences its properties during processes like aluminum casting or heat treatment.
Heat Treatment
Techniques such as annealing or quenching change not only the aluminum but also the distribution of heat within the material and its density. If you take aluminum, heat it and then cool it rapidly, it causes the distribution of atoms to be random which reduces the density. Heat treatments are employed and controlled strictly to increase carrying capacity and corrosion resistance.
Pressure
Under high pressures, aluminium is compressed and hence its density is slightly raised. However, if you are working with aluminum in areas of high pressure like deep sea or aerospace, then it is imperative that this compression is taken into consideration precisely in order to be able to guarantee the structural, and functional reliability of the said material.
Cooling Rate
The rate of cooling aluminum affects its density. Quenching, for example, can retain specific phases or defects, which slightly narrows density. Since the rate of cooling is slower, then grain coalescence will also be uniform. One should always ensure that cooling rates are controlled in casting or heat treatment if they want to attain predictable density results.
Precipitation Hardening
Precipitation hardening makes aluminum stronger by creating fine particles in the metal though, at the same time it changes the density. When you are implementing this process you will realize that this marginally raises density because of the extra weight of the precipitates and at the same time enhances the strength of the material without compromising the weight.
Work Hardening
Work hardening is achieved every time you deform the aluminum through mechanical techniques. This increases its density by provoking the atoms to put themselves nearer collectively. When aluminium is formed through processes such as bending or hammering then its density increases and so does the strength but at the cost of ductility.
Aging
Ageing whether natural or artificial promotes formation of precipitates in the aluminium alloys. This causes the density to increase slightly as the alloy reaches a point of stabilization. When you require enhanced strength or protection against corrosion, the right heat treating can enhance these characteristics while incurring a minimal change in density.
Deformation History
The previous deformations of aluminum can also affect the current density of the container. When using recycled or remanufactured aluminum, it is crucial to know that this material undergoes changes in the microstructure when it is deformed repeatedly; this process bends the material, making the metal more dense closer to the frame.
Surface Coatings
Coating of aluminum component often involves use of extra layers such as anodizing or painting, while these processes do not change density of the bulk aluminum, they do add extra mass to the component. You should take into account the mass of coatings in precision applications where any variation in the total mass might be critical.
Hydration State
Incorporated into this is the concept of moisture – the specific condition under which aluminum may become corroded. Upon oxidation, aluminum forms an oxide layer on its surface which slightly increases the density of the material. This is ordinarily inconsequential but may be relevant in places where precision of weight is of significance.
Comparison with Other Metals’ Densities
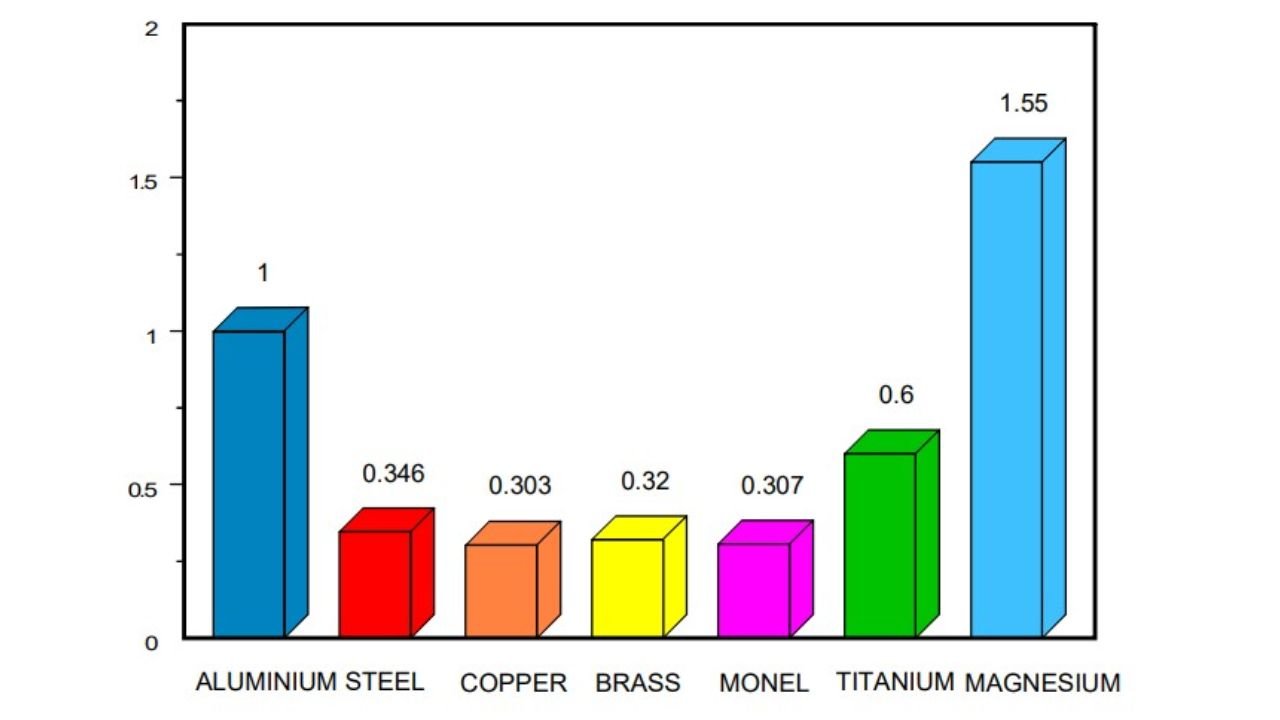
| Sr. No. | Metal | Density (g/cm³) | Melting Point (°C) | Boiling Point (°C) | Crystal Structure | Other Properties |
| 1 | Aluminum | 2.7 | 660 | 2519 | Face-Centered Cubic (FCC) | Excellent corrosion resistance, lightweight, good conductor of heat and electricity. |
| 2 | Copper | 8.96 | 1083 | 2567 | Face-Centered Cubic (FCC) | High ductility, malleability, and thermal conductivity. Used in electrical wiring, plumbing, and alloys. |
| 3 | Iron | 7.87 | 1538 | 2862 | Body-Centered Cubic (BCC) | Strong, durable, and magnetic. Widely used in construction, machinery, and tools. |
| 4 | Lead | 11.34 | 327 | 1740 | Face-Centered Cubic (FCC) | Soft, malleable, and toxic. Used in batteries, ammunition, and plumbing. |
| 5 | Magnesium | 1.74 | 650 | 1107 | Hexagonal Close-Packed (HCP) | Lightweight, strong, and resistant to corrosion. Used in aerospace, automotive, and electronics industries. |
| 6 | Nickel | 8.91 | 1455 | 2732 | Face-Centered Cubic (FCC) | Hard, strong, and resistant to corrosion. Used in alloys, batteries, and catalysts. |
| 7 | Titanium | 4.51 | 1668 | 3287 | Hexagonal Close-Packed (HCP) | Strong, lightweight, and corrosion-resistant. Used in aerospace, medical implants, and industrial applications. |
| 8 | Zinc | 7.14 | 419 | 907 | Hexagonal Close-Packed (HCP) | Malleable, ductile, and resistant to corrosion. Used in alloys, batteries, and galvanizing. |
Density Variations Among Aluminum Grades!
| Sr. No. | Grade | Density (g/cm³) | Primary Use | Aluminum Content (%) | Typical Alloying Elements | Weldability | Strength (MPa) | Corrosion Resistance |
| 1 | 1100 Series | 2.7 | General-purpose sheet, plate, tubing | 99.0-99.7 | No significant alloying elements | Excellent | Low | Good |
| 2 | 2024 Grade | 2.8 | Aircraft parts, structural components | 92.0-94.0 | Copper, magnesium, manganese | Good | High | Moderate |
| 3 | 3003 Grade | 2.7 | Deep drawing, stamping, cookware | 95.0-97.0 | Manganese | Excellent | Moderate | Good |
| 4 | 5052 Grade | 2.7 | Marine applications, chemical tanks | 97.0-98.0 | Magnesium | Excellent | Moderate | Excellent |
| 5 | 6061 Grade | 2.7 | Structural components, extrusion, welding | 96.0-98.0 | Magnesium, silicon | Excellent | Moderate-high | Good |
| 6 | 7075 Grade | 2.8 | Aircraft parts, structural components | 90.0-92.0 | Zinc, copper, magnesium | Good | Very high | Moderate |
Common Aluminum Alloys and Their Compositions!
| Sr. No. | Alloy | Composition | Applications | Density (g/cm³) | Properties |
| 1 | 6061-T6 | Al-Mg-Si | Structural parts, aerospace components, furniture, machinery | 2.7 | Excellent strength-to-weight ratio, good corrosion resistance, weldable |
| 2 | 7075-T6 | Al-Zn-Cu-Mg | Aerospace components, structural parts, bicycle frames, firearms | 2.8 | Highest strength among aluminum alloys, good fatigue resistance, weldable |
| 3 | 2024-T3 | Al-Cu-Mg | Aerospace components, structural parts, marine applications | 2.8 | High strength, good fatigue resistance, weldable |
| 4 | 3003-H14 | Al-Mn | Food containers, cookware, architectural applications | 2.7 | Good corrosion resistance, soft and ductile, easily formed |
| 5 | 5052-H32 | Al-Mg | Marine applications, chemical equipment, food containers | 2.7 | Excellent corrosion resistance, good formability, moderate strength |
| 6 | 1100-H14 | Pure aluminum | Electrical conductors, heat exchangers, food containers | 2.7 | Excellent electrical and thermal conductivity, soft and ductile, easily formed |
| 7 | 5083-H32 | Al-Mg | Marine applications, chemical equipment, transportation | 2.7 | Excellent corrosion resistance, good formability, moderate strength |
| 8 | Al-Li alloys | Al-Li | Aerospace components, structural parts | 2.5 | Low density, high modulus of elasticity, good fatigue resistance |
| 9 | Al-Si alloys | Al-Si | Automotive parts, engine blocks, pistons | 2.6 | Good castability, high wear resistance, good thermal conductivity |
| 10 | Al-Cu alloys | Al-Cu | Electrical conductors, heat exchangers, marine applications | 2.7 | Good electrical and thermal conductivity, high strength, good corrosion resistance |
| 11 | Al-Mg alloys | Al-Mg | Marine applications, chemical equipment, transportation | 2.7 | Excellent corrosion resistance, good formability, moderate strength |
| 12 | Al-Zn alloys | Al-Zn | Aerospace components, structural parts, marine applications | 2.8 | High strength, good fatigue resistance, weldable |
| 13 | Al-Fe alloys | Al-Fe | Automotive parts, engine blocks, pistons | 2.7 | High strength, good wear resistance, good thermal conductivity |
| 14 | Al-Ni alloys | Al-Ni | High-temperature applications, aerospace components | 2.7 | High temperature strength, good oxidation resistance |
| 15 | Al-Sc alloys | Al-Sc | Aerospace components, structural parts | 2.7 | Excellent strength-to-weight ratio, good fatigue resistance |
Revealing Density Dominant Applications and Calculation Techniques
How to Calculate the Density of Aluminum?
Measurement of Mass
First, weigh your sample of aluminum. The results should be very accurate and for this, a precise scale of measurement is required. The real demsity value for the aluminum lies around 2.7 g/cm^3 which make it lighter. For instance, a 50-gram sample is said to be compact, a feature associated with high mass/volume ratio.
Volume Measurement
For determination of the volume of the aluminum, one can place it in a liquid that the volume of the aluminum displaces. The free electron density of aluminum is 18 x 10²³ electrons per cubic meter. An actual small cube having dimension 2 cm is made from aluminum and its volume is 8 cm³. This will explain how volume affects material behaviour.
Density Formula
Aluminum’s density formula is straightforward: divide mass by volume. For instance, if you have 40 grams of aluminium occupying 15 cm³, then you divide 40 by 15, then you will have approximately 2.7 g/cm^3. By applying this formula, it becomes very easy and accurate to determine the density of any aluminum sample.
Unit Conversion
There exists a variation in units of density depending on the type of industry in question. In the U. S, it is expressed in pounds per cubic feet (lb/ft³); equivalent to 168 lb/ft³ for aluminum. To convert from 2.7 g/cm^3 to lb/ft³, you will use a conversion factor of 62.43. Regular conversions help to make the collaboration with the business counterparts in other countries seamless.
Archimedes’ Principle
Some of the methods that can be used for determining aluminum’s volume and therefore density are based on Archimedes’ principle. Place a piece of aluminum in water and measure the amount of water that displaced. Finally, divide the mass of the aluminum you got in the previous step by this volume to arrive at the density. The expected result is approximately 2.7 g/cm^3.
Hydrostatic Weighing
For hydrostatic weighing, weigh the aluminum in air and then with the aluminum submerged in water. Subtract the submerged weight of the object from the air weight of the object to get the buoyant force. Next, divide the weight of the air by the buoyancy force to get the density that is usually about 2. undefined
Pycnometer Method
Aluminum density is accurately measured using rather a unique equipment known as pycnometer that is actually a sort of flask. Initially, the pycnometer was weighed full of water; afterwards, the water was replaced by aluminum and the weighing was made again. Thus, having compared the two weights and taking into account the density of water, it is possible to determine the density of aluminum.
Gas Displacement
Gas displacement involves using helium or nitrogen to determine the volume that an aluminum occupies. The process involves evacuating air from the chamber, then the introduction of the gas, and the registration of the change in the pressure. Mass divided by volume will give the density, which is a favourite method for dealing with precise industries.
X-ray Crystallography
In X-ray crystallography, you study the diffraction pattern that comes from aluminum atoms. This method offers information at the Atomic Structure level. If you know unit cell volume and use Avogadro’s number you calculate density, which is generally close to 2.7 g/cm^3 It is a complex and accurate method.
Applications Where Aluminum Density Calculation Matters
Material selection
Density calculations can help you select materials carefully. Density of approx 2.7 g/ cm^3 for aluminum makes it lighter in comparison to steel that has 7.8 g/ cm^3 density. By utilizing lightweight components, fuel usage is reduced and overall performance enhanced in automotive sector.
Structural analysis
In construction, the density of materials such as aluminum is important so that strength and weight can be integrated well. They prefer to use 6061-T6 aluminum for the frame that has a density of 2. 7 g/cm³ which shows that the material is strong yet relatively light. Knowing these values can help you create safer and more efficient structural designs.

Fluid dynamics
In fluid dynamics examination the aluminum oxide is distinguished with the density 3.95 to 4.1 2.7 g/ cm^3. This affords better opportunity to interact with liquids and gases owing to its higher density. This makes it useful in high performance applications such as in rocket parts where the fluid behavior around the materials is important.
Thermal management
Aluminum has a density of 2.7 g/ cm^3 at 25°C and has a major bearing when it comes to heat transfer. They are also a good conductor of heat, and are very useful in areas of electronics and automotive applications. You can also use it in automobiles, computers, or any other system where overheating can be dangerous to the engine, equipment, or other sensitive components.
Packaging design
This makes it ideal for packaging since Aluminums density is 2.7 g/ cm^3. It is light, hence makes it easier to transport it from one place to another and creates a barrier against light, oxygen and bacteria. That is why you use it in products such as soda cans and food packaging; it is both safe and efficient.
Acoustics
Aluminum plays a critical role in the acoustic design. In terms of acoustic properties, sound waves can pass through it at 6,420 meters per second, providing good sound quality. It also gives the optimum density for speaker diaphragms which produce better sounds and less distortion thus favored by audiophiles.
Material handling
Compared to other materials, a distinct advantage of using aluminum is evident in material handling where it boasts high strength-to-weight ratio. Commonly used alloys, such as 6063, can produce structures for conveyor systems that are stable and light at the same time. Low density makes it easy to transport and Aluminium’s ability to prevent sparking makes it safe to use in areas where explosions are likely to occur.
Process optimization
Achieving faster production processes is on the go since aluminum has a density that would increase production speeds. Its melting point is at 660.3°C enables quick casting and molding. Aluminum allows to speed up the process of cooling; however, the use of alloys like 7075, which is lightweight but also hard, which is suitable for manufacturing, will be greatly beneficial.
Inventory management
Because aluminum is not very dense, you can place products on top of one another without thinking about the cumulative weight. It is highly resistant to corrosion so it provides longer storage without deforming the product. Consequently, understanding this property in the workshop helps to optimise vacant space and increase the shelf-life of stored items, which makes it a reasonable option in regards to inventory management.
Conclusion
Finally, it can be stated that the knowledge of the density of aluminum is important for determining the proper material for different applications given different sectors. Thus, this guide has focused on the basic density of aluminium, its attributes, and the comparison of the aluminium density with other metal types. The information is useful in understanding the relation between temperature, composition of alloys, and processing methods and density and thus becomes useful in choosing the right aluminum for a given application. Knowing density enables effective performance for various uses including aerospace and normal products. These points should be well understood to improve your performance in decision making whenever you are working on a project that requires the use of aluminum.
Read More:
Melting Point of Aluminum – Variations, Alloys, Comparison




One Response